Introduction:
Globally 2.3 million children died in the first 28 days of life in 2022. There are approximately 6500 newborn deaths every day, amounting to 47% of all child deaths under the age of 5 years. Most neonatal deaths (75%) occur during the first week of life, and about 1 million newborns die within the first 24 hours. Among neonates, the leading causes of death include premature birth, birth complications (birth asphyxia/trauma), neonatal infections and congenital anomalies, which collectively account for almost 4 in every 10 deaths in children under 5 years of age. It is worth noting that although the rates for the leading causes of neonatal deaths have declined globally since 2000, they accounted for the same proportion of under-5 deaths – 4 in 10 – in 2000 and 2022. The vast majority of newborn deaths take place in low and middle-income countries. Access to and availability of quality health care continues to be a matter of life or death for mothers and newborns globally.
Nonetheless, global data demonstrating the effectiveness and real-world impact of maternal immunization remain limited and not widely accessible.
Just as an example, in 2018, approximately 25 000 newborns died from neonatal tetanus, a 97% reduction from 1988 when an estimated 787 000 newborn babies died of tetanus within their first month of life, before universal tetanus vaccination was implemented during pregnancy.
In addition, pregnancy is a known risk factor for developing more severe illness following infections such as Influenza, COVID-19, Hepatitis A, Hepatitis E, and others. Therefore, maternal immunization should aim to protect not only the newborn but also the mother.
Mechanisms in which vaccination during pregnancy protects the neonate:
Several studies suggest that the newborn’s immune system is ‘untrained’ or ‘immature,’ possibly due to the prioritization of nutrient resources for the rapid development of the central nervous system, among other proposed theories. What is well established, however, is that neonates are significantly more susceptible to infections compared to older children and adults. In this context, immediate protection through maternally derived antibodies—via maternal immunization—may represent the most effective early-life protective strategy.
Maternal and fetal circulations are separated by a layer of villous trophoblastic cells. Immunoglobulin G (IgG) is actively transported across the syncytiotrophoblast via neonatal Fc receptor (FcRn)-mediated transcytosis. This process is further supported by Hofbauer cells and IgG-containing vesicles, which help protect the antibodies from proteolytic degradation. Altogether, this finely tuned mechanism represents a remarkable natural immunological system designed to provide passive immunity to the fetus—a true biological work of art.
Maternal IgA antibodies induced by vaccination can be transferred to the newborn through breast milk, providing immune protection at mucosal surfaces, particularly in the infant’s gastrointestinal tract. Evidence supports that this form of passive immunity occurs following maternal vaccination against pathogens such as influenza, COVID-19, and others.
Protecting the mother from severe illness—such as influenza or COVID-19—not only safeguards her well-being but also indirectly protects the newborn from a range of complications, including preterm birth and even neonatal death. Ensuring maternal health is, therefore, a critical strategy for improving neonatal outcomes.
Herd effect: Vaccinating the mother can contribute to indirect protection of the infant through reduced transmission risk among close contacts and caregivers. This herd effect lowers the likelihood that the infant—or other infants in the household or community—will be exposed to infection.
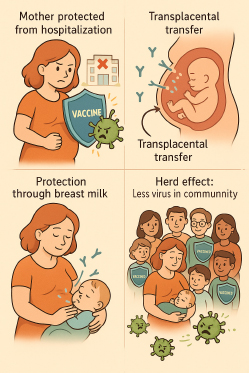
Figure 1, mechanisms in which vaccination during pregnancy protects the neonate:
Safety:
Pharmacovigilance is a pivotal aspect of all vaccination programs, but in the context of pregnancy, safety considerations are even more critical due to the need to protect both the mother and the fetus. This heightened concern may have contributed to the slower development and approval of vaccines specifically for use during pregnancy.
Live attenuated vaccines are generally contraindicated during pregnancy due to theoretical concerns regarding fetal risk. However, available evidence does not indicate significant adverse effects on the fetus following inadvertent administration of these vaccines. Consequently, the contraindication of vaccines such as measles-mumps-rubella (MMR) during pregnancy is largely precautionary. Notably, unintentional immunization with MMR-containing vaccines during pregnancy is not considered an indication for pregnancy termination.
In summary, the use of live attenuated vaccines during pregnancy requires a highly individualized and cautious benefit-risk assessment. Key considerations include the mother’s risk of developing severe disease after a confirmed or high-risk exposure—such as an unvaccinated, immunocompromised pregnant woman in close contact with a confirmed measles case—and the gestational age, given the heightened risk of teratogenic effects during the first trimester.
There is one vaccine that has been associated with a slightly increased risk of preterm birth—the respiratory syncytial virus (RSV) vaccine. However, this risk is extremely low, and the substantial benefit of protecting the infant from severe RSV disease, including a significantly reduced risk of hospitalization during the first six months of life, outweighs the potential concern. Current evidence supports the continued use of the RSV vaccine in pregnancy as a valuable tool for neonatal protection.
Current vaccines available during pregnancy:
- Diphtheria and tetanus: Either as DT or DTaP.
- Pertussis: As DTaP.
- Influenza.
- COVID-19.
- RSV.
- Hepatitis-A (not routinely recommended, but preterm labor occurs in more than 60% of cases in addition to other potential complications).
Future candidates for vaccination during pregnancy:
- Group B Streptococcus (maybe soon to be implemented).
- Cytomegalovirus.
- Zika virus.
- Lassa fever.
- Hepatitis-E.
Vaccine uptake during pregnancy, a barrier:
Numerous studies have demonstrated variable uptake of vaccines during pregnancy. In the United Kingdom, for example, coverage of maternal influenza and pertussis vaccination declined during the COVID-19 pandemic—from 71% to 61% for influenza, and from 44% to 35% for pertussis. Surveys of both healthcare providers and the general population have revealed concerningly low awareness and acceptance of maternal vaccines, particularly for pathogens like influenza and COVID-19, which significantly impact maternal and neonatal health. These findings highlight persistent gaps in knowledge regarding the safety and effectiveness of approved vaccines during pregnancy, underscoring the need for targeted education and outreach.
Conclusion:
Maternal immunization should be recognized as a pivotal public health intervention worldwide. To fully realize its potential, several key challenges must be addressed: expanding the inclusion of pregnant individuals in clinical trials, strengthening post-approval safety surveillance through robust cohort studies, enhancing education to improve awareness and acceptance, and ensuring equitable investment—particularly in low- and middle-income countries (LMICs). Addressing these barriers is essential not only to reduce neonatal and infant mortality, but also to protect the health of pregnant women and those of reproductive age.
Bibliography
- Male V, Jones CE. Vaccination in pregnancy to protect the newborn. Nat Rev Immunol. 2025 Apr 23. doi: 10.1038/s41577-025-01162-5.
- Omer SB, Clark DR, Madhi SA, Tapia MD, Nunes MC, Cutland CL, Simões EAF, Aqil AR, Katz J, Tielsch JM, Steinhoff MC, Wairagkar N; BMGF Supported Maternal Influenza Immunization Trials Investigators Group. Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials. Lancet Respir Med. 2020 Jun;8(6):597-608. doi: 10.1016/S2213-2600(19)30479-5.
- Vygen-Bonnet S, Hellenbrand W, Garbe E, von Kries R, Bogdan C, Heininger U, Röbl-Mathieu M, Harder T. Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review. BMC Infect Dis. 2020 Feb 13;20(1):136. doi: 10.1186/s12879-020-4824-3.
- Kontovazainitis CG, Katsaras GN, Gialamprinou D, Mitsiakos G. Covid-19 vaccination and pregnancy: a systematic review of maternal and neonatal outcomes. J Perinat Med. 2023 Feb 17;51(7):823-839. doi: 10.1515/jpm-2022-0463.
- Röbl-Mathieu M, Kunstein A, Liese J, Mertens T, Wojcinski M. Vaccination in Pregnancy. Dtsch Arztebl Int. 2021 Apr 16;118(15):262-268. doi: 10.3238/arztebl.m2021.0020.
- Etti M, Calvert A, Galiza E, Lim S, Khalil A, Le Doare K, Heath PT. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol. 2022 Apr;226(4):459-474. doi: 10.1016/j.ajog.2021.10.041.
- Rand CM, Olson-Chen C. Maternal Vaccination and Vaccine Hesitancy. Pediatr Clin North Am. 2023 Apr;70(2):259-269. doi: 10.1016/j.pcl.2022.11.004.
- Razai MS, Mansour R, Ravindran P, Freeman S, Mason-Apps C, Morris J, Majeed A, Ussher M, Hargreaves S, Oakeshott P. Facilitators and barriers to vaccination uptake in pregnancy: A qualitative systematic review. PLoS One. 2024 Apr 19;19(4):e0298407. doi: 10.1371/journal.pone.0298407.
- Arora M, Lakshmi R. Vaccines – safety in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2021 Oct;76:23-40. doi: 10.1016/j.bpobgyn.2021.02.002.
- WHO: Updates on monitoring safety during pregnancy and breastfeeding projects: PERLA and COVID-19 pregnancy cohort study. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/pregnancy-and-lactation/vaccines.
- Chittajallu LVS, Kaku R, Kondadasula P, Lim JY, Zhumabekova A. Safety and Efficacy of Vaccines During Pregnancy: A Systematic Review. Cureus. 2025 Jan 9;17(1):e77176. doi: 10.7759/cureus.77176.
- WHO: Newborn mortality. March 2024. https://www.who.int/news-room/fact-sheets/detail/newborn-mortality.
- WHO: Tetanus. July 2024. https://www.who.int/news-room/fact-sheets/detail/tetanus.
- CDC Guidelines for vaccinating pregnant women. July 2024. https://www.cdc.gov/vaccines-pregnancy/hcp/vaccination-guidelines/index.html.
- Healthline: Can Hepatitis A affect pregnancy? July 2023. https://www.healthline.com/health/hep-a-pregnancy.
- WHO: Immunization, vaccines and biologicals: Group B Streptococcus. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/group-b-streptococcus-(gbs).
- Kirsty Le Doare, Virginia Benassi, Marco Cavaleri, Godwin Enwere, Birgitte Giersing, David Goldblatt, Paul Heath, Joachim Hombach, Richard Isbrucker, Kostas Karampatsas, Shabir A. Madhi, Annelies Wilder Smith. Clinical and regulatory development strategies for GBS vaccines intended for maternal immunisation in low- and middle-income countries. Vaccine. 2025: 127131. https://doi.org/10.1016/j.vaccine.2025.127131.
- AJMC: Infant RSV Hospitalization Rates Drop in First Season With Widespread Preventive Product Use. https://www.ajmc.com/view/infant-rsv-hospitalization-rates-drop-in-first-season-with-widespread-preventive-product-use.
- CDC: Respiratory Virus Hospitalization Surveillance Network (RESP-NET). https://www.cdc.gov/resp-net/dashboard/index.html.
- Raut S, Apte A, Srinivasan M, Dudeja N, Dayma G, Sinha B, Bavdekar A. Determinants of maternal influenza vaccination in the context of low- and middle-income countries: A systematic review. PLoS One. 2022 Jan 26;17(1):e0262871. doi: 10.1371/journal.pone.0262871.







