Introduction:
Arboviruses (arthropod-borne viruses) are a group of viruses transmitted to humans through the bites of infected arthropods—primarily mosquitoes, but also ticks, fleas, and gnats. There are over 130 known arboviruses that can cause disease in humans, many of which pose serious public health threats, particularly in tropical and subtropical regions.
The majority of human arboviral infections are caused by viruses belonging to three main genera:
1. Flavivirus
- Yellow fever virus
- West Nile virus
- Zika virus
- Dengue virus
- Japanese encephalitis virus
- Zika virus
2. Togavirus
- Ross River virus
- Eastern equine encephalitis virus
- Western equine encephalitis virus
- Chikungunya virus
3. Bunyavirus
- California encephalitis virus
- La Crosse virus
- Jamestown Canyon virus
- Oropouche virus (Orthobunyavirus)
While arboviruses are primarily transmitted through the bites of infected insects, several other modes of transmission have been documented, including:
- Blood transfusion
- Organ transplantation
- Sexual contact
- Vertical transmission (from mother to child during pregnancy or childbirth)
Understanding these viruses and their transmission dynamics is critical for effective surveillance, prevention, and outbreak response strategies.
Human to human transmission of most arboviruses through casual, everyday contact has not been documented.
Disease burden:
Particularly dengue, yellow fever, chikungunya and Zika viruses are all current public health threats in tropical and sub-tropical areas where approximately 3.9 billion people live. The frequency and magnitude of outbreaks of these arboviruses, particularly those transmitted by Aedes mosquitoes, are increasing globally, fueled by the convergence of ecologic, economic and social factors.
Recent global data reveal a substantial surge in dengue cases worldwide. Over 7.6 million infections have been reported, including 3.4 million laboratory-confirmed cases, 16,000 classified as severe, and approximately 3,000 associated deaths. While the Chikungunya virus (CHIKV) and Zika virus (ZIKV) also continue to circulate, their case numbers remain significantly lower, with an estimated 250,000 and 7,000 reported cases, respectively.
In the Americas, the Pan American Health Organization/World Health Organization (PAHO/WHO) have reported a total of 3,125,386 arboviral infections. Of these, dengue accounts for the vast majority—approximately 90% (2,811,452 cases)—followed by Chikungunya at 8.8% (273,685 cases), and Zika at 1.3% (40,249 cases).
The Global Arbovirus Initiative:
The Global Arbovirus Initiative, launched on 31 March 2022, represents a comprehensive, cross-sectoral effort spearheaded by the World Health Organization (WHO). It brings together the WHO Health Emergencies Program, the Department of Control of Neglected Tropical Diseases, and the Immunization, Vaccines and Biologicals Department, in collaboration with an expanding network of international, multisectoral partners.
The initiative is built around key strategic pillars that provide a framework for its objectives and priority actions. These focus on monitoring risk, strengthening pandemic prevention and preparedness, enhancing early detection and response capabilities, and fostering a global coalition of stakeholders.
By integrating efforts across sectors, the initiative aims to enhance coordination, communication, capacity building, and research. Ultimately, it seeks to bolster global preparedness and response systems to mitigate the growing threat posed by emerging and re-emerging arboviruses with epidemic and pandemic potential.
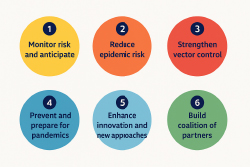
The Global Arbovirus Initiative is structured around six strategic pillars:
- Monitor risk and anticipate outbreaks,
- Reduce the risk of local epidemics,
- Strengthen vector control measures,
- Prevent and prepare for pandemics,
- Enhance innovation and promote new approaches, and
- Build a coalition of partners to support coordinated action.
Prevention:
Aside from vaccination, the most effective way to prevent arboviral infections is by avoiding insect bites, particularly in regions with high rates of transmission.
Personal Protection Against Insect Bites:
- Individuals can reduce their risk of bites by:
- Using insect repellent on exposed skin and clothing
- Wearing long-sleeved shirts and long pants when outdoors
- Tucking pants into socks to prevent insects from reaching the skin
- Wearing light-colored clothing, which makes insects easier to spot
Reducing Mosquito Breeding Sites:
Mosquitoes breed in standing water. Minimizing stagnant water in and around the home is a key strategy to reduce mosquito populations.
Practical steps include:
- Removing or regularly emptying containers that collect rainwater (e.g., buckets, flowerpots, birdbaths)
- Drilling drainage holes in the bottom of outdoor containers
- Cleaning gutters to ensure proper water flow and drainage
- Avoiding the use of old tires in landscaping, which can collect water
- Maintaining pools and hot tubs with proper cleaning and chlorination
While these measures have demonstrated effectiveness, they remain insufficient to fully control these diseases in endemic regions and during outbreaks. Therefore, the continued development and deployment of vaccines against these viruses are essential to achieving sustainable disease control.
Vaccines against dengue virus (DENV), CHIKV and ZIKV:
DENV:
The chimeric live-attenuated vaccine CYD-TDV (Dengvaxia®), developed by Sanofi Pasteur, was first licensed in 2015 for use in Mexico, the Philippines, Brazil, and later in over 20 additional countries. The vaccine is based on a yellow fever 17D virus backbone engineered to express dengue virus (DENV) structural genes, creating a chimeric construct designed to induce immunity against all four DENV serotypes. However, the vaccine’s efficacy has been shown to vary depending on several factors, including DENV serotype, the individual’s serostatus at the time of vaccination, geographic region, and age. Importantly, CYD-TDV is indicated only for individuals who are seropositive for dengue at the time of vaccination. Its use in DENV-naive individuals is not recommended due to the increased risk of severe dengue illness following subsequent natural infection—a phenomenon attributed to antibody-dependent enhancement (ADE).
Qdenga® (TAK-003) is a live-attenuated, tetravalent dengue vaccine developed by Takeda. The vaccine uses a DENV-2 genetic backbone to construct chimeric viruses representing all four dengue serotypes. Specifically, the structural pre-membrane (prM) and envelope (E) genes of DENV-2 were replaced with those from DENV-1, DENV-3, and DENV-4 to generate the respective serotype-specific components, while DENV-2 remained in its native form. Pooled data from Phase 2 and 3 clinical trials conducted across multiple countries in individuals aged 4 to 60 years demonstrate that TAK-003 is well tolerated regardless of age, sex, or baseline dengue serostatus. In a Phase 3 trial involving children and adults living in dengue-endemic areas, TAK-003 demonstrated over 70% efficacy during the first-year post-vaccination, independent of prior dengue exposure. Further results from a three-year Phase 3 trial conducted in eight countries across Asia and Latin America revealed sustained protection. Two doses of TAK-003 provided a cumulative efficacy of 62.0% against virologically confirmed dengue and 83.6% against hospitalized dengue. Efficacy by serotype in dengue-exposed individuals ranged from 52.3% against DENV-3 to 80.4% against DENV-2. Among seronegative participants, protection was considered adequate for DENV-1 and DENV-2, but insufficient against DENV-3. The low incidence of DENV-4 in the study population prevented a reliable assessment of efficacy against that serotype. Some of the countries where Qdenga® has been approved include Brazil, Argentina, Indonesia, Thailand, and also recommended as a traveler’s vaccine in Canada, the UK, and many other European countries.
A live-attenuated tetravalent dengue vaccine (LATV) represents another promising strategy, developed by the National Institute of Allergy and Infectious Diseases (NIAID/NIH). The LATV candidates, TV003 and TV005, consist of four recombinant live-attenuated dengue virus components: rDEN1Δ30, rDEN2/4Δ30, rDEN3Δ30/3Δ31, and rDEN4Δ30. These formulations have demonstrated a robust and balanced immune response after a single dose, inducing trivalent or tetravalent neutralizing antibodies in most participants. Although a single primary dose has shown strong immunogenicity, the potential benefit of a booster regimen is still under evaluation, particularly in individuals with pre-existing dengue immunity. The Butantan Institute® in Brazil, in collaboration with the NIH, is producing and testing this vaccine under the name Butantan-DV® in dengue-endemic populations with high baseline seropositivity. The vaccine is currently undergoing Phase 3 clinical trials. In a two-year follow-up analysis, a single dose of Butantan-DV® demonstrated 89.5% efficacy against symptomatic DENV-1 and 69.6% against DENV-2, regardless of participants’ baseline serostatus. Due to the absence of DENV-3 and DENV-4 cases during the study period, efficacy against these serotypes could not be assessed.
V181 is a live attenuated quadrivalent vaccine currently being investigated for the prevention of dengue disease caused by any of the four dengue virus types (DENV-1, DENV-2, DENV-3, and DENV-4). V181 is designed to be a single-dose vaccination and is being studied in individuals to provide protection against dengue, including severe forms, whether the individuals have been previously infected with the dengue virus or had no prior infections. Merck (NYSE: MRK), known as MSD outside of the United States and Canada, just announced the initiation of the MOBILIZE-1 Phase 3 clinical trial evaluating the safety, immunogenicity and efficacy of a single dose of V181. Both Merck and Instituto Butantan’s investigational vaccines are derived from materials licensed from the U.S. National Institutes of Health (NIH).
Other dengue vaccine candidates—including subunit, inactivated, DNA, and mRNA-based platforms—are currently in various stages of clinical development, representing innovative approaches aimed at improving safety, immunogenicity, and accessibility.
CHIKV:
VLA1553, developed by Valneva®, is a single-dose, live-attenuated vaccine designed to provide broad protection against all circulating strains of chikungunya virus (CHIKV). The vaccine is derived from the LR2006 OPY1 strain, which belongs to the East/Central/South African (ECSA) genotype. A key attenuation feature is a 61–amino acid deletion in the nsP3 gene, essential for viral replication, which significantly reduces the virus’s virulence while maintaining immunogenicity.
In clinical trials, VLA1553 demonstrated excellent immunogenicity, with 98.9% of participants achieving seroconversion following a single dose. The immune response was durable, with 96.3% retaining protective levels of neutralizing antibodies up to 180 days post-vaccination. Long-term data showed that neutralizing antibodies remained above protective thresholds for up to two years, with no serious long-term adverse events reported.
The vaccine was generally well tolerated. Reported side effects were mostly mild to moderate and included headache, fever, arthralgia, and myalgia.
In November 2023, VLA1553 was approved by the U.S. FDA under the trade name Ixchiq®, initially indicated for travelers. It was subsequently authorized in Canada and Europe in June 2024. While approval for adolescents aged ≥12 years is underway, both the European Medicines Agency (EMA) and the U.S. FDA have paused recommendations for adults over 65 years pending further investigation into rare severe adverse events reported in this age group, including some during the outbreak on La Réunion Island. It is important to note that a causal link between these events and the vaccine has not yet been established. Regulatory review of Ixchiq® is ongoing in the United Kingdom and Brazil, where a Phase 3 clinical trial in adolescents (VLA1553-321) is currently underway. Additionally, a Phase 2 clinical trial evaluating the safety and immunogenicity of two dose levels of a single-shot vaccine in 304 children showed that antibody levels remained high at six months. The full dose demonstrated both superior immunogenicity and a comparable safety profile, supporting its selection for the pivotal Phase 3 trial.
Virus-like particles (VLPs) represent a major advancement in vaccine development, offering strong immunogenicity while maintaining an excellent safety profile due to the absence of viral genetic material. A notable VLP-based chikungunya vaccine candidate, VRC-CHKVLP059-00-VP, was developed using structural polyprotein genes—including capsid, E3, E2, 6K, and E1—from the Senegal 37997 strain of CHIKV. Now marketed under the trade name Vimkunya® by Bavarian Nordic, this vaccine was approved by the U.S. FDA in February 2025 and subsequently authorized by the European Medicines Agency (EMA) for individuals aged 12 years and older. Like other leading candidates, Vimkunya® is administered as a single-dose vaccine, making it a promising option for broad deployment in endemic and non-endemic settings alike.
Other CHIKV vaccine candidates—including live-attenuated, viral vector, and mRNA-based platforms—are currently in various stages of clinical development, reflecting a diverse and evolving landscape in the pursuit of effective chikungunya prevention.
ZIKV:
Currently, there is no approved vaccine for Zika virus; however, multiple candidates are advancing through clinical development. One notable example is VLA1601 (Valneva®), an inactivated vaccine platform that is currently in Phase 2 clinical trials. In addition to inactivated vaccines, a diverse array of platforms is being explored, including live attenuated, mRNA, chimeric, DNA, subunit, viral vector, and virus-like particle (VLP) vaccines, reflecting a broad and innovative approach to Zika virus prevention.
Conclusions:
Arboviruses, particularly those transmitted by mosquitoes, are responsible for millions of infections and thousands of hospitalizations and deaths worldwide. While non-vaccine preventive measures remain important and can be effective, they are insufficient to fully control these infections and their associated diseases. Vaccines against DENV and CHIKV have demonstrated high efficacy and safety profiles, offering significant promise in disease control. However, for ZIKV and many other arboviruses, vaccine development is still in progress, with encouraging but preliminary results.
The WHO Global Arbovirus Initiative, structured around six strategic pillars, represents a critical step forward in the comprehensive control of arboviral diseases. Yet, as with all public health challenges, achieving equitable progress is essential. This includes equitable access and investment in education, vaccine manufacturing, surveillance systems, laboratory capacity, infrastructure scale-up, regulatory frameworks, and political commitment, among many other factors. Only through addressing these multifaceted needs can we hope to effectively combat arbovirus threats worldwide.
Bibliography
- WHO Global Arbovirus Initiative. Accessed: June 3, 2025. https://www.who.int/initiatives/global-arbovirus-initiative.
- Huang YS, Higgs S, Vanlandingham DL. Emergence and re-emergence of mosquito-borne arboviruses. Curr Opin Virol. 2019 Feb;34:104-109. doi: 10.1016/j.coviro.2019.01.001.
- Tajudeen YA, Oladunjoye IO, Mustapha MO, Mustapha ST, Ajide-Bamigboye NT. Tackling the global health threat of arboviruses: An appraisal of the three holistic approaches to health. Health Promot Perspect. 2021 Dec 19;11(4):371-381. doi: 10.34172/hpp.2021.48.
- Wang M, Liu K, Guo D, Lv Y, Wang X. Arbovirus Infections and Epigenetic Mechanisms; a Potential Therapeutic Target. Rev Med Virol. 2025 May;35(3):e70033. doi: 10.1002/rmv.70033.
- Pereira CAM, Mendes RPG, Silva PGD, Chaves EJF, Pena LJ. Vaccines Against Urban Epidemic Arboviruses: The State of the Art. Viruses. 2025 Mar 6;17(3):382. doi: 10.3390/v17030382.
- Bernasconi V, Kristiansen PA, Whelan M, Román RG, Bettis A, Yimer SA, Gurry C, Andersen SR, Yeskey D, Mandi H, Kumar A, Holst J, Clark C, Cramer JP, Røttingen JA, Hatchett R, Saville M, Norheim G. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020 Jan;63(1):65-73. doi: 10.1007/s00103-019-03061-2.
- Ahola T, Couderc T, Ng LF, Hallengärd D, Powers A, Lecuit M, Esteban M, Merits A, Roques P, Liljeström P. Therapeutics and vaccines against chikungunya virus. Vector Borne Zoonotic Dis. 2015 Apr;15(4):250-7. doi: 10.1089/vbz.2014.1681.
- Kok BH, Lim HT, Lim CP, Lai NS, Leow CY, Leow CH. Dengue virus infection – a review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023 Jan 15;324:199018. doi: 10.1016/j.virusres.2022.199018.
- Poland GA, Kennedy RB, Ovsyannikova IG, Palacios R, Ho PL, Kalil J. Development of vaccines against Zika virus. Lancet Infect Dis. 2018 Jul;18(7):e211-e219. doi: 10.1016/S1473-3099(18)30063-X.
- Côrtes N, Lira A, Prates-Syed W, Dinis Silva J, Vuitika L, Cabral-Miranda W, Durães-Carvalho R, Balan A, Cabral-Marques O, Cabral-Miranda G. Integrated control strategies for dengue, Zika, and Chikungunya virus infections. Front Immunol. 2023 Dec 18;14:1281667. doi: 10.3389/fimmu.2023.1281667.
- Prasad V, Kaslow DC, Yang S. Pausing Chikungunya Vaccination and Accelerated Approval. JAMA. Published online June 05, 2025. doi:10.1001/jama.2025.9393.
- Valneva Reports Positive Six-Month Antibody Persistence and Safety Phase 2 Results in Children for its Single-Shot Chikungunya Vaccine IXCHIQ®. Accessed June 5, 2025. https://valneva.com/press-release/valneva-reports-positive-six-month-antibody-persistence-and-safety-phase-2-results-in-children-for-its-single-shot-chikungunya-vaccine-ixchiq/.
- Merck Initiates Phase 3 Study Evaluating Dengue Vaccine Candidate. Last accessed June 12, 2025. https://www.merck.com/news/merck-initiates-phase-3-study-evaluating-dengue-vaccine-candidate/